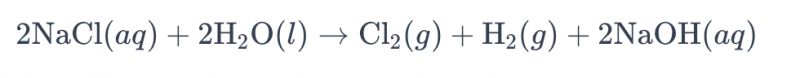The process of electrolyzing a brine solution using titanium electrodes to produce chlorine is commonly referred to as "electrolysis of brine." In this process, titanium electrodes are employed to facilitate the oxidation reaction of chloride ions in the brine, leading to the generation of chlorine gas. The overall chemical equation for the reaction is as follows:
In this equation, chloride ions undergo oxidation at the anode, resulting in the production of chlorine gas , while water molecules are reduced at the cathode, yielding hydrogen gas. Additionally, hydroxide ions undergo reduction at the anode, forming hydrogen gas and sodium hydroxide.
The choice of titanium electrodes is due to titanium's excellent corrosion resistance and conductivity, allowing it to undergo the reaction stably during electrolysis without corrosion. This makes titanium electrodes an ideal choice for the electrolysis of brine.
The electrolysis of saline water typically requires an external power source to provide energy for the electrolytic reaction. This power source is usually a direct current (DC) power supply because electrolytic reactions necessitate a consistent direction of current flow, and a DC power supply can deliver a constant current direction.
In the process of electrolyzing saline water to generate chlorine gas, a low-voltage DC power supply is commonly employed. The voltage of the power supply depends on specific reaction conditions and equipment design, but generally ranges between 2 to 4 volts. Additionally, the current intensity of the power supply is a crucial parameter that needs to be determined based on the size of the reaction chamber and the desired production yield.
In summary, the choice of power supply for the electrolysis of saline water depends on the specific requirements of experiments or industrial processes to ensure efficient reaction and the attainment of the desired products.
Post time: Jan-16-2024