In the world, everything has its pros and cons. The progress of society and the improvement of people's living standards inevitably lead to environmental pollution. Wastewater is one such issue. With the rapid development of industries such as petrochemicals, textiles, papermaking, pesticides, pharmaceuticals, metallurgy, and food production, the total discharge of wastewater has increased significantly worldwide. Moreover, wastewater often contains high concentrations, high toxicity, high salinity, and high color components, making it difficult to degrade and treat, leading to severe water pollution.
To deal with the large volumes of industrial wastewater generated daily, people have employed various methods, combining physical, chemical, and biological approaches, as well as utilizing forces such as electricity, sound, light, and magnetism. This article summarizes the use of "electricity" in the electrochemical water treatment technology to address this issue.
Electrochemical water treatment technology refers to the process of degrading pollutants in wastewater through specific electrochemical reactions, electrochemical processes, or physical processes within a particular electrochemical reactor, under the influence of electrodes or an applied electric field. Electrochemical systems and equipment are relatively simple, occupy a small footprint, have lower operating and maintenance costs, effectively prevent secondary pollution, offer high controllability of reactions, and are conducive to industrial automation, earning them the label of "environmentally friendly" technology.
Electrochemical water treatment technology includes various techniques such as electrocoagulation-electroflotation, electrodialysis, electroadsorption, electro-Fenton, and electrocatalytic advanced oxidation. These techniques are diverse and each has its own suitable applications and domains.
Electrocoagulation-Electroflotation
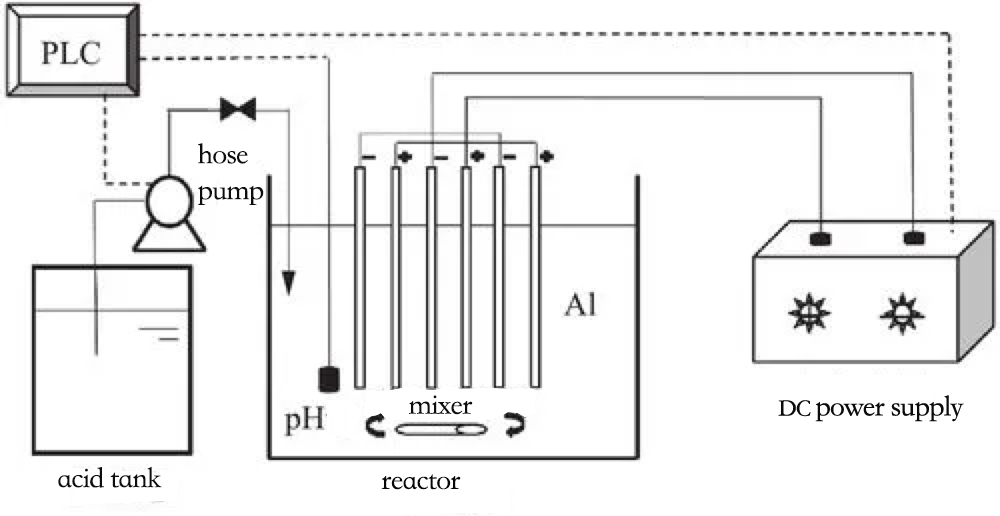
Electrocoagulation, in fact, is electroflotation, as the coagulation process occurs concurrently with flotation. Therefore, it can be collectively referred to as "electrocoagulation-electroflotation."
This method relies on the application of an external electric voltage, which generates soluble cations at the anode. These cations have a coagulating effect on colloidal pollutants. Simultaneously, a substantial amount of hydrogen gas is produced at the cathode under the influence of the voltage, which helps the flocculated material rise to the surface. In this way, electrocoagulation achieves the separation of pollutants and purification of water through anode coagulation and cathode flotation.
Using a metal as the soluble anode (typically aluminum or iron), the Al3+ or Fe3+ ions generated during electrolysis serve as electroactive coagulants. These coagulants work by compressing the colloidal double layer, destabilizing it, and bridging and capturing colloidal particles through:
Al -3e→ Al3+ or Fe -3e→ Fe3+
Al3+ + 3H2O → Al(OH)3 + 3H+ or 4Fe2+ + O2 + 2H2O → 4Fe3+ + 4OH-
On one hand, the formed electroactive coagulant M(OH)n is referred to as soluble polymeric hydroxo complexes and acts as a flocculant to rapidly and effectively coagulate colloidal suspensions (fine oil droplets and mechanical impurities) in wastewater while bridging and linking them to form larger aggregates, speeding up the separation process. On the other hand, colloids are compressed under the influence of electrolytes such as aluminum or iron salts, leading to coagulation through the Coulombic effect or adsorption of coagulants.
Although the electrochemical activity (lifespan) of electroactive coagulants is only a few minutes, they significantly affect the double layer potential, thus exerting strong coagulation effects on colloidal particles or suspended particles. As a result, their adsorption capacity and activity are much higher than chemical methods involving the addition of aluminum salt reagents, and they require smaller amounts and have lower costs. Electrocoagulation is not affected by environmental conditions, water temperature, or biological impurities, and it does not undergo side reactions with aluminum salts and water hydroxides. Therefore, it has a wide pH range for treating wastewater.
Additionally, the release of tiny bubbles on the cathode surface accelerates the collision and separation of colloids. The direct electro-oxidation on the anode surface and the indirect electro-oxidation of Cl- into active chlorine have strong oxidative capabilities on soluble organic substances and reducible inorganic substances in water. The newly generated hydrogen from the cathode and oxygen from the anode have strong redox capabilities.
As a result, the chemical processes occurring inside the electrochemical reactor are extremely complex. In the reactor, electrocoagulation, electroflotation, and electrooxidation processes all occur simultaneously, effectively transforming and removing both dissolved colloids and suspended pollutants in water through coagulation, flotation, and oxidation.
Xingtongli GKD45-2000CVC Electrochemical DC POWER SUPPLY
Features:
1. AC Input 415V 3 Phase
2. Forced air cooling
3. With ramp up function
4. With amper hour meter and time relay
5. Remote control with 20 meter control wires
Product images:


Post time: Sep-08-2023




